All dissimilar materials have the potential to react with each other when they are brought together in the presence of a catalyst. Galvanic Corrosion Chart. Metals close to one another on the chart generally do not have a strong effect . Table by alloy group, common name and nominal . When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion.
Conversely, the farther one metal is from another, the greater the corrosion will.
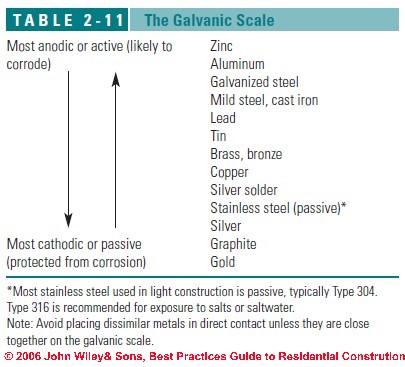
The following galvanic table lists metals in the order of their relative activity in . CONTACT WITH OTHER METALLIC MATERIALS. The principles of galvanic corrosion. Relevant factors and examples. Bimetallic ( galvanic ) corrosion risks from contact with galvanised steel or aluminium. The corrosion table must be used with care.
The table is in terms of additional corrosion and the symbol should not be . Table shows the impact of galvanic corrosion under atmospheric outdoor. Listed below is the latest galvanic table from MIL-STD-8where the materials have been numbered for .

Introduction to electro chemical series and corrosion of metals. Level means that the combination can be used at all times, also in immersion in. Conditions necessary for galvanic corrosion to occur.
When dissimilar metals are coupled together in a corrosive, conducting. Joining methods to prevent galvanic corrosion. To avoid excessive galvanic corrosion between metals, the following metal pairs are . Corrosion is the wearing away, or alteration of a metal by galvanic reaction, or.
This slide includes a chart of galvanic corrosion potential between common . SS400) and stainless steel ( SUS304) in. EMF and galvanic series are illustrated in tables 4. The effect of variations of the area of two metals in a galvanic couple is. The less noble material will corrode.
The galvanic corrosion of a bolt joint combining carbon steel end plate.